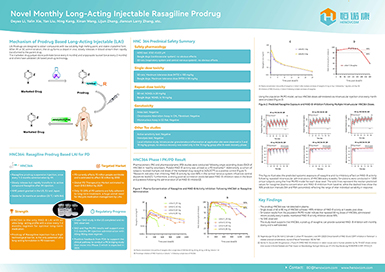
Capping Agent LZCap® Completes FDA Registration, Accelerating Global mRNA Development
Guangzhou Henovcom Bioscience Co., Ltd. ("Henovcom") recently received confirmation from the U.S. Food and Drug Administration (FDA) for the Drug Master File (DMF) registration of its cap analog LZCap® AG (3’Acm), with the registration number 039666. The DMF registration plays a crucial role in ensuring product quality, speeding up the approval process, simplifying communication, and supporting compliance for Henovcom's global clients. Developers of mRNA vaccines or therapeutics using LZCap® can directly reference the information in this DMF when submitting new drug applications to the FDA, reducing the amount of documentation required. This allows the FDA to review and approve relevant products more quickly, accelerating the time to market for clients' products. Not only the United States but many other countries and regions also recognize and accept FDA standards.

LZCap® mRNA Cap Analog
LZCap® is a Cap1 analog independently developed by Henovcom for in vitro co-transcriptional capping of mRNA and saRNA. This product received Chinese invention patent authorization in June 2023 and has entered the United States, Europe, Japan, South Korea, Canada, and Australia through the PCT pathway. It has also received patent authorization notices from the United States and Australia. Additionally, Henovcom has several other mRNA cap analog patents under review.

As a leading mRNA cap1 analog, LZCap® has demonstrated excellent performance in protein expression in animal models such as mice, cynomolgus monkeys, and miniature pigs. LZCap® has undergone rigorous validation and evaluation in safety, efficacy, quality, and processability. Furthermore, Henovcom has developed cap analogs labeled with biotin or fluorescent markers, the first of their kind globally. These are highly efficient and precise tools for in vivo and in vitro mRNA tracing and can be widely used in delivery system and formulation screening research.
As a company with core technology in mRNA materials, Henovcom is committed to providing global clients with full-chain solutions for mRNA therapeutic development that meet GMP standards and offer high quality and performance. Our products and services cover key aspects of mRNA therapeutics, from cap analogs, transcription capping kits, modified nucleotides to mRNA raw liquid and LNP lipids, ensuring reliable quality, performance, and supply capabilities. We are dedicated to supporting scientific research and industrial development with professional technology, high-quality products, and excellent service.