A Prodrug-based Ultra-long Acting Injectable 505(b)(2) New Drug About to Enter Pivotal Clinical Trial
Guangzhou Henovcom Bioscience Co., Ltd. (Henovcom) recently reached agreement with the U.S. Food and Drug Administration (FDA) regarding the relative bioavailability (rBA) clinical study of HNC364, Henovcom’s proprietary ultra-long-acting innovative Parkinson's disease drug. This significant advancement marks a key milestone in the clinical development of HNC364.
According to the consensus reached between Henovcom and the FDA at the End-of-Phase 2 (EOP2) clinical development meeting, HNC364 will be advanced directly to a pivotal Phase 3 confirmatory clinical study after the completion of the rBA clinical study. These two clinical studies will ultimately support the New Drug Application (NDA) of HNC364 in the US.
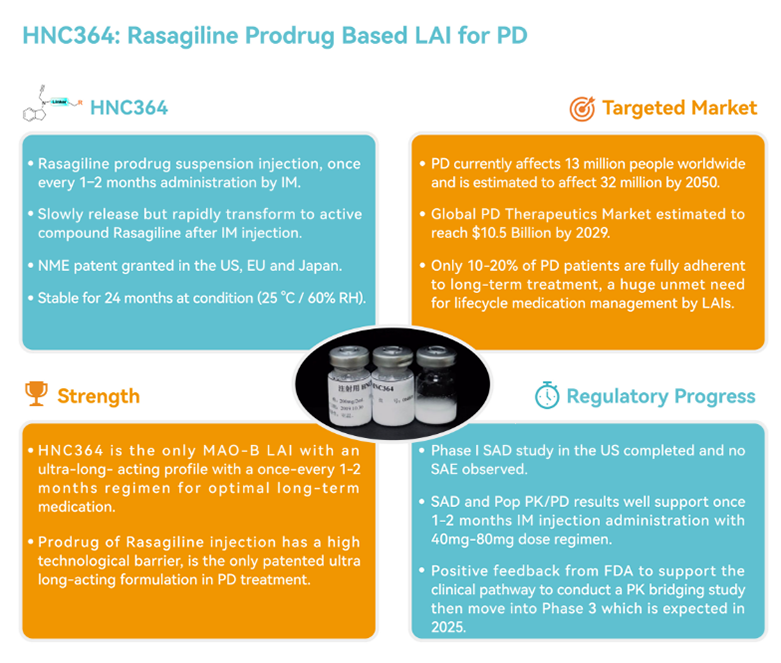
HNC364 is a 505(b)(2) modified new drug developed by Henovcom based on its proprietary " Prodrug-based Long-acting Microcrystal Technology Platform". It is the first ultra-long-acting Parkinson's disease treatment in China to receive FDA approval for clinical studies and is designed to be administered once every 1-3 months.
The product has already filed patent applications for the prodrug new molecular entity in key global regulatory markets and has successfully obtained patent grants in China, the United States, Europe (UK, France, Germany, Italy, and Spain) and Japan.
Prodrug-based Long-acting Microcrystal Technology: Breaking Traditional Treatment Barriers
Henovcom’s proprietary Prodrug-based Long-acting Microcrystal Technology involves structural modifications to existing daily oral medications, designing prodrug compounds with independent intellectual property rights. These compounds are then formulated into microcrystal injectables. When injected subcutaneously or intramuscularly, the formulation is slowly and continuously released and rapidly converted to the active drug, thereby exerting its therapeutic effect.
Technical and Clinical Advantages
1. Addressing the Long-term Medication Trend for Chronic Diseases: Long-acting formulations have become the globally recognized best solution for the pharmaceutical industry and FDA.
2. Improved Patient Adherence and Optimized Treatment Experience: Reducing the frequency of administration greatly improves patient convenience while minimizing the risk of potential side effects.
3. Significant Manufacturing Advantages: Compared to traditional microsphere formulations, the microcrystal injectable process is stable, scalable, and easier to control, effectively eliminating the risk of "burst release".
4. Shorter Clinical Development Cycle and Lower Drug Development Risk: The clinical research pathway is simplified, with the potential to bypass Phase 2 trials and proceed directly to pivotal Phase 3 trials. Several marketed long-acting prodrug products have successfully validated the safety and efficacy of this development approach.
5. Industry-leading Technological Barriers: With strong molecular design capabilities and years of experience in the prodrug field, Henovcom is ensuring that HNC364 and its prodrug pipeline remain at the forefront of the industry.
The successful progress of HNC364 not only offers Parkinson’s disease patients a more convenient and effective treatment option but also further strengthens Henovcom’s leading position in the field of long-acting innovative drugs.
Henovcom is seeking partners for out-licensing or co-development for the global market, please contact: BD@henovcom.com.
More data is detailed in the poster: