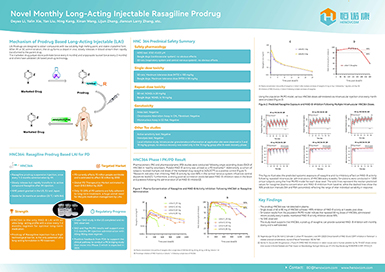
GMP Workshop of Nucleotide Raw Material of Henovcom officially put into operation
In the morning of January 3rd, the opening ceremony of the GMP workshop of Nucleotide Raw Materials of Henovcom was successfully held. This marks the official opening of Henovcom’s production workshop, which officially started construction in July 2022 and took nearly half a year to finish. The workshop has a building area of 2300 square meters with multiple functional areas throughout the entire professional process, including research and development laboratories, pilot scale workshops, production workshops (D-level clean areas), QC (quality control) laboratories, etc. It has the capacity to develop and produce high-efficiency core capping raw materials for LZCap® from multiple grams to multiple kilograms per batch, which will facilitate the industrialization of mRNA vaccines and drugs.


Chairman of Henovcom, Dr. Larry (Jiancun) Zhang, together with shareholder representatives, and representatives of the nucleotide project team attended the opening ceremony and jointly witnessed this historical moment. At the ceremony, Dr. Larry (Jiancun) Zhang stated that the completion and operation of the workshop is an important milestone in the development and production of Henovcom's nucleotide raw material business. The company's GMP-compliant production and quality management system will contribute to the worldwide development of mRNA vaccines and drugs, cell therapy, and gene editing technologies. Meanwhile, it will promote the rapid and high-quality development of Henovcom's nucleotide raw material business, and quickly enter the global market.