Breakthrough in patent lockdown! Henovcom's self-developed mRNA cap analogues have been patented for protection
Recently, Henovcom Biosciences Co., Ltd. received the Notice of Patent Grant from the China National Intellectual Property Administration on "Compounds for RNA capping and their applications (Patent No.: ZL202210973168.5)". This invention patent relates to the compound structure and application of a cap1 cap analog for co transcription capping. Furthermore,this is the first authorized patent in China for the structure of mRNA co-transcription capping analogs. Besides Chinese invention patents, Henovcom has started to expand its presence in other major market countries through the PCT approach.

Figure 1. Notice of Grant of Invention Patent Right sent by China National Intellectual Property Administration
One-step co transcription is currently the preferred method for mRNA preparation, but the capping material has become a bottleneck in the upstream mRNA supply chain in China due to patent protection. Therefore, Henovcom has developed a co-transcription capping product with independent intellectual property rights, LZCap ® , and successfully overcome the problem of "neck blockage". The upstream key raw material of vaccines, drugs and therapies based on mRNA technology is nucleoside triphosphate. The co-transcriptional capping raw material and modified nucleoside triphosphate raw material with high technical barriers account for more than 50% of the cost of raw materials for mRNA vaccines, and the capping raw material accounts for about 35% of the cost of the whole mRNA. This type of high-end raw material is like AI's GPU chip, which is the core component of mRNA vaccines, drugs, and therapies. However, the preparation process of high-end co-transcription capping raw materials is very complex and has patent protection. Domestic enterprises used to rely only on imports, and the instability of the global supply chain and high costs are the major challenges faced by domestic mRNA vaccine and drug production enterprises, becoming a "bottleneck" technology that restricts the development of mRNA vaccine- and drug-related fields in China. LZCap ® developed by Henovcom is taking the lead in breaking foreign patent monopolies.
LZCap ® is currently the world's leading hat analogue. Compared to the foreign monopoly of the capping reagent CleanCap, LZCap ® can generate mRNA in one step within a few hours and demonstrates huge advantages, including high capping rate, high transcriptional yield, and low immunogenicity. Importantly, the expression efficiency of structural mRNA containing LZCap ® is significantly higher than that of mRNA containing CleanCap. Meanwhile, due to the optimized production process and strict quality control system of Henovcom, LZCap ® have significant competitive advantages while ensuring high quality, which will bring significant potency and cost advantages to downstream mRNA products.
The patent authorization of cap analogues with independent intellectual property rights will accelerate the high-quality development of the entire mRNA industry chain in China. With significant advantages of safety and speed, mRNA therapy is a major industrialization direction of biomedicine in the future. It has been widely used in vaccine, cell therapy, protein substitution, genome editing, regenerative medicine and other important fields. The research and development of multiple pathogenic vaccines based on mRNA, CAR-T and TCR-T cell therapy, antigen substitution in tumor immunity, genome editing and protein substitution have entered a rapid development stage. Henovcom's nucleotide raw material technology platform is currently a leading technology platform in the world. With 2300m2 GMP compliant production workshop built and ISO9001 certification, Henovcom is capable of producing various "high-precision" and "bottleneck" upstream raw materials. This will accelerate the rapid development of cutting-edge technologies such as mRNA vaccines, cell therapy, and protein replacement therapy downstream of the industrial chain. It is of great significance for promoting the high-quality development of China's biopharmaceutical industry and moving towards the forefront of global biopharmaceutical innovation.

Figure 2. Henovcom’s ISO9001 Certification
[About co-transcription capping technology and cap1 cap analogues]
Transcription is the process of producing RNA from DNA template. In this process, immature RNA is first generated using DNA as a template, and then it undergoes a series of post transcriptional modifications in the nucleus to form mature mRNA. mRNA is the template for subsequent translation of amino acids and produce proteins. Mature mRNA contains five structures: 5 'cap structure (5' cap), 5 'untranslated region (5' UTR), open reading frame encoding antigen, 3 'untranslated region (3' UTR) and a PolyA tail. Among them, the cap structure is crucial for mRNA. The cap structure can not only regulate the cleavage maturation of mRNA, help RNA transcripts pass through the selective channels of the nuclear membrane, but also protect mRNA from degradation by exonucleases, collaborate with translation initiation factor proteins, recruit ribosome, and assist ribosome to bind to mRNA, and then translation could begin.
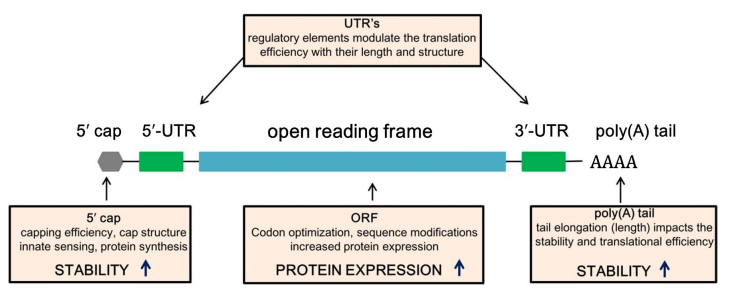
Figure 3. Mature mRNA contains five structural units
Therefore, in the development of mRNA products, the in vitro modification process is very crucial. The mRNA vaccine or drug also needs to form a cap structure in order to be stably expressed in vivo. There are three forms of cap structures in vivo: cap0, cap1, and cap2. The traditional method of modifying mRNA in vitro (cowpox virus capping enzyme method) can add cap0 cap to the 5 'end of mRNA, and then use methyltransferase to process it to get cap1 cap. The mRNA vaccine or drug needs to form Cap 1 structure to be stably expressed in vivo (Cap2 exists in 10%-15% of eukaryotic cell, but its function has not been effectively verified). However, the drawback of this method is that the use of cowpox virus capped enzymes is expensive and the cost of enzymatic hydrolysis is high. In addition, due to the introduction of additional proteins by enzyme capping, the preparation process is complex and requires multiple purification, adding QA/QC detection items. The new generation of "co-transcription capping" LZCap® is to directly transcribe in vitro with cap analogues to generate capped cap1 cap structure mRNA, facilitating the "one pot" mRNA preparation. The process is simple, and can also rapidly improve the production capacity of mRNA vaccines and drugs.